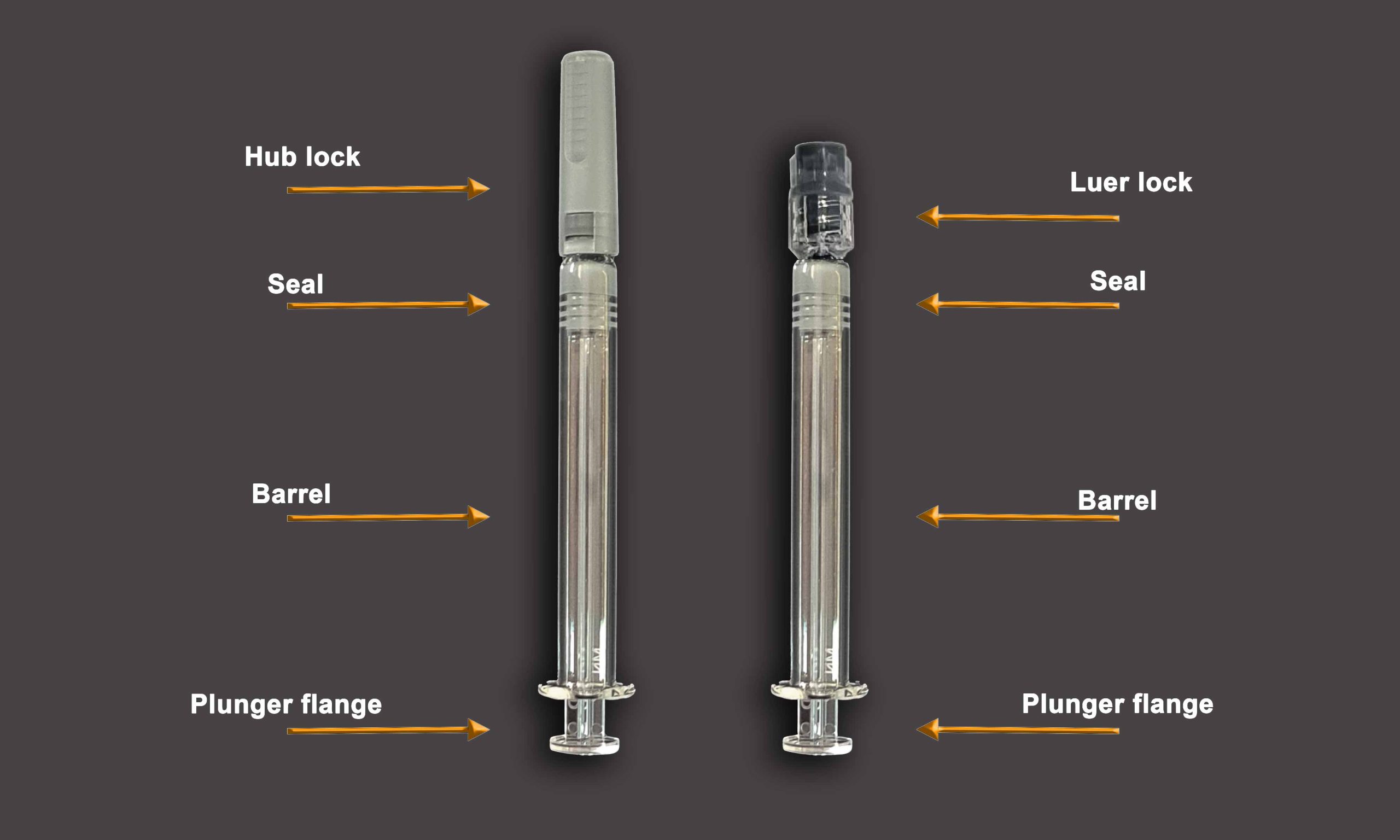
Medicine and Packaging:
An ampoule (also ampul and ampule) is a small sealed vial used to contain and preserve a sample, usually a solid or liquid. Ampoules are made of glass. Modern ampoules are most commonly used to contain pharmaceuticals and chemicals that must be protected from air and contaminants. They are hermetically sealed by melting the thin top with an open flame and usually opened by snapping off the neck. The space above the chemical may be filled with an inert gas before sealing. The walls of glass ampoules are usually sufficiently strong to be brought into a glovebox without any difficulty.
Glass ampoules are more expensive than bottles and other simple containers, but there are many situations where their superior imperviousness to gases and liquids, as well as an all-glass interior surface, are worth the extra cost. Examples of chemicals sold in ampoules include injectable pharmaceuticals, air-sensitive reagents like tetrakis(triphenylphosphine)palladium(0), hygroscopic materials like deuterated solvents and trifluoromethanesulfonic acid, and analytical standards. Ampoules can be pressurized, have air evacuated from them, and have the air replaced with other gases, often inert ones.
The radiopharmaceutical Xenon-133 is often packaged in glass ampoules. Specially-shaped glass ampoules have long been used for samples of gaseous elements, such as all of the Column 18 Noble Gases except radon (mainly because it is radioactive with a half-life less than half a week). Special thick-walled quartz and fluorite ampoules under high pressure containing fluorine and chlorine liquefied by the high pressure have also been developed. Teflon ampoules have been designed, based on the concept of the Teflon jug for high-molarity hydrofluoric acid, to contain chemicals that would corrode and/or ignite glass, contaminate themselves, corrode, or disintegrate metal containers where the reagent does not passivate the metal by rapidly forming a layer of a new inert compound on the metal surface reliably and predictably.
Photosensitive chemicals, like many 14-dihydromorphinone opioids (such as hydromorphone and oxymorphone), various silver salts, and so on, can be packaged in ampoules of smoked glass, glass with chemicals added during manufacturing that filter out ultraviolet and other types of light, or be made with an opaque top and bottom (usually painted with opaque paint) and the rest of the ampoule wrapped in thick paper.
Historic Ampoules :
Historically, ampoules were used to contain a small sample of a person’s blood after death, which was entombed alongside them in many Christian catacombs. It was originally believed that only martyrs were given this burial treatment, though it is suspected to have been a widely practiced tradition.
Production and Usage :
Modern glass ampoules are produced industrially from short lengths of glass tubing, shaped by heating with gas torches and gravity in automated production lines. Computer vision techniques are usually employed for quality control.
Filling :
The filling and sealing of ampoules may be done by automated machinery on an industrial scale, or by hand in small-scale industries and laboratories. Ampoule filling machines can be categorized into three categories: automatic, semi-automatic, and manual (hand-operated) machines.
Blank ampoules can be purchased from scientific glass supply houses and sealed with a small gas torch. A Schlenk line may be used for sealing under inert atmospheres. The procedure involves purging nitrogen before and after filling liquid into ampoules in order to remove atmospheric air from inside the ampoules.